NANOLETTERS 2009 Vol. 9, No. 8 3007-3011
Ganga Periyasamy and F. Remacle
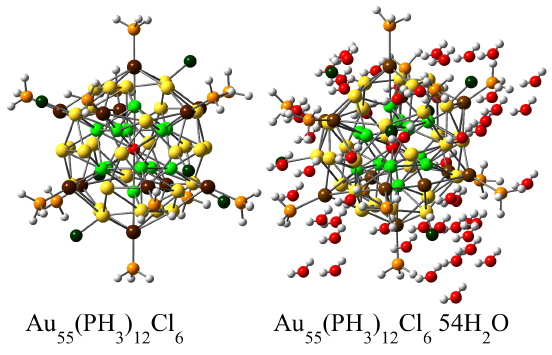
The electronic properties of the neutral, positively and negatively charged bare Au55, passivated Au55(PH3)12, Au55(PH3)12Cl6, and solvated Au55(PH3)12Cl6 54 H2O clusters are studied using density functional theory. The presence of Cl atoms in the ligand shell favors a non-metallic behavior while a more metallic behavior is induced by explicit solvation of Au55(PH3)12Cl6 with water molecules. The trends observed in the electronic properties upon ligation and solvation are in agreement with experimental studies.